Inhaltsverzeichnis
Werbung
Verfügbare Sprachen
Verfügbare Sprachen
Quicklinks
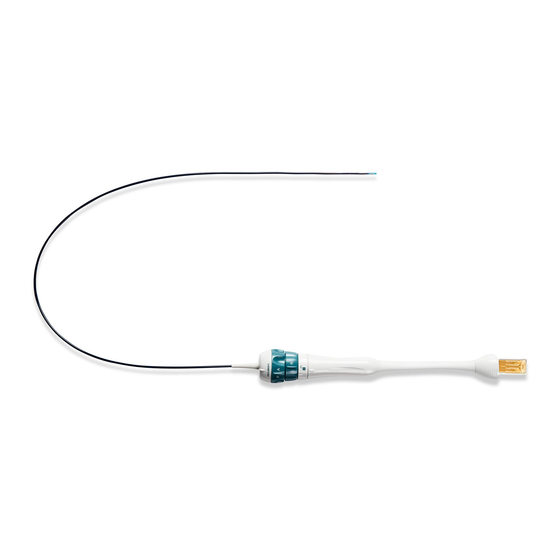
ACUSON AcuNav Ultrasound Catheter
The symbols on the label, catheter, connector, and Directions for Use are defined in
the ACUSON AcuNav Ultrasound Catheter User Manual.
CONVENTIONS
Convention
Description
Catheter
ACUSON AcuNav 8F diagnostic ultrasound catheter
ACUSON AcuNav 10F diagnostic ultrasound catheter
Connector
SwiftLink catheter connector
DESCRIPTION
Each catheter is sterile, disposable, and licensed for single use only. The
ACUSON AcuNav ultrasound catheter distal end has an ultrasound transducer
providing 2-D imaging. A steering mechanism controls the image plane orientation
through four-way articulation of the tip.
The catheter is intended for intracardiac and intraluminal visualization of cardiac and
great vessel anatomy and physiology as well as visualization of other devices in the
heart.
Refer to your ultrasound system user documentation for information about catheter
and connector compatibility with your ultrasound system.
CONTRAINDICATIONS
WARNING: The catheter is not for use in coronary vessels or fetal tissue. Using
the catheter in coronary vessels or fetal tissue can cause patient injury.
Use of the catheter is contraindicated under conditions where the cardiac
catheterization process would cause unacceptable risk to the patient.
Contraindicated conditions include, but are not limited to, cases where vascular
access is inadequate. Known contraindicated conditions include: sepsis, major
coagulation abnormalities, presence of any intracardiac thrombus, presence of
class IV angina or heart failure, deep vein thrombosis, and significant peripheral
vascular disease or abnormalities.
USE GUIDELINES
Caution: In the United States of America, federal law restricts this device for
sale or use by, or on the order of, a physician.
Use of the catheter is only by or under the supervision of physicians well trained in
cardiac catheterization. Preferably, physicians using the catheter have been trained
in the placement and use of intracardiac imaging devices and in the interpretation of
the resulting ultrasound images.
DIRECTIONS FOR USE
WARNING: Do not resterilize, reprocess, or reuse the catheter. Reusing the
catheter can damage the structural integrity of the catheter and can result in a
contaminated catheter. Use of a contaminated catheter can cause patient
infection. Use of a contaminated catheter can transmit infectious disease from
one patient to another. Use of a contaminated catheter can result in patient
illness or death.
WARNING: Completely read and understand the ACUSON AcuNav Ultrasound
Catheter User Manual and your ultrasound system user documentation before
you attempt to connect the catheter to any ultrasound system and operate the
catheter. Failure to completely read and understand the ACUSON AcuNav
Ultrasound Catheter User Manual and your ultrasound system user
documentation can result in patient injury.
The Directions for Use are intended as a review of catheter procedures. The
Directions for Use do not include essential background and instructional information
that is necessary for successful use of the catheter. Refer to the ACUSON AcuNav
Ultrasound Catheter User Manual and your ultrasound system user documentation
for complete catheter use directions.
Physicians interested in further training in the use of the catheter should contact
their catheter distributor for training opportunities.
Adverse Reactions
Adverse events related to cardiac catheterization have been documented. Adverse
events related to cardiac catheterization include (but are not limited to): femoral
artery or vein injury, thrombosis, pseudoaneurysm, cardiac perforation, air
embolism, pulmonary embolism, myocardial infarction, valve or structural cardiac
damage, cardiac tamponade, pneumothorax, hemothorax, arteriovenous (AV)
fistula, stroke, and death.
Interfering Substances or Devices
It is imperative that you are aware of the pacemaker or implantable cardioverter-
defibrillator (ICD) needs of the patient. If use of the catheter interferes with the
function of the patient's implantable device, immediately discontinue use of the
catheter.
10030119-ABS-001-12-12
Procedure Preparation
WARNING: Do not use the connector if the connector appears damaged in any
way. Using a damaged connector can result in patient or user injury. Contact
your connector distributor.
WARNING: Do not immerse the connector. Moisture trapped between the
connector and the catheter can damage the connector and/or the catheter,
causing possible patient or user injury or death. Do not use the connector if the
connector appears wet. Contact your connector distributor.
WARNING: Do not use the catheter if the packaging is open or damaged. Using
a catheter that has been stored in an open or damaged package can result in
patient or user injury. Contact your catheter distributor.
WARNING: Do not use the catheter if the catheter appears damaged in any
way. Using a damaged catheter can result in patient or user injury. Contact your
catheter distributor.
Before you begin the preparation procedures, power on the ultrasound system.
To prepare the catheter and connector for use in an ultrasound exam:
1. Inspect the connector for damage.
2. Inspect the sterile package and the catheter prior to use.
3. Using proper sterile technique, remove the catheter from the sterile package.
Place the catheter in a sterile working area.
4. Inspect the entire catheter for damage.
5. Rotate the steering knobs. The steering function should be smooth. The
catheter tip should flex in a corresponding direction up to 160°.
Note: If the catheter tip does not return to the neutral position after you release
the steering knobs, ensure that the tension control knob is completely released.
Release the tension by rotating the tension control knob completely in a
counterclockwise direction.
6. Position the steering knobs in the neutral position by aligning the marks on the
steering knobs to the marks on the housing.
7. Connect the system connector to the ultrasound system.
8. Slip the sterile sheath over the catheter interconnect tab until the sheath is fully
seated on to the catheter handle.
9. Lift the lever on the connector. Slip the connector on to the catheter interconnect
tab until the connector is fully mated with the catheter handle. Push the lever
down, locking the catheter to the connector.
10. Carefully slip the sterile sheath over the connector. Cover enough of the
connector so the connector is out of the sterile field.
11. Verify that the imaging screen appears.
During the Procedure
WARNING: Do not use excessive force to advance or withdraw the catheter.
Using excessive force can result in patient injury or death. Ensure that the two
steering knobs are in the neutral position and the tension control knob is
released before advancing or withdrawing the catheter. If you encounter strong
resistance during catheter articulation, discontinue the procedure. Identify and
address the cause of the resistance before resuming the procedure. Withdraw
and redirect the catheter as needed.
Caution: Excessive bending or kinking of the catheter can damage internal
wires and/or distal tip articulating capabilities.
To conduct an ultrasound exam using the catheter:
1. Create a vascular access with a catheter introducer (hemostatic) large enough
to accommodate the catheter with heparinized saline.
2. Before advancing or withdrawing the catheter, ensure that the steering knobs
are in the neutral position and that the tension control knob is released.
3. Advance the catheter into the vasculature through the catheter introducer.
Fluoroscopy can aid in advancing the catheter into the heart.
4. When the catheter is inside the heart, use the steering knobs to direct the
ultrasound transducer to visualize the target cardiac anatomy.
Procedure Conclusion
WARNING: Regard the used catheter, introducer, and sheath as biohazardous,
infectious waste. Dispose of the used catheter, introducer, and sheath according
to local, state, and regional regulations for biohazardous waste. Observe local,
state, and regional regulations for the disposal of electrical and electronic
equipment. Wear appropriate protective gear while handling the used catheter,
introducer, and sheath. Failure to dispose of the used catheter properly can
result in harm to the environment and to anyone who comes in contact with the
used catheter.
WARNING: Do not resterilize, reprocess, or reuse the catheter. The catheter is
disposable and is licensed for single use only. Reusing the catheter can
damage the structural integrity of the catheter and can result in loss of proper
electrical and mechanical function. Use of a damaged catheter can result in
patient injury or death.
To end an ultrasound exam using the catheter:
1. Before you withdraw the catheter, ensure that the steering knobs are in the
neutral position and that the tension control knob is released.
2. Withdraw the catheter from the patient.
3. Dispose of the catheter, introducer, and sheath.
Disposing of the Packaging Materials
Local laws and regulations may require manufacturers to collect and dispose of
packaging materials at no cost to the customer. Contact your catheter or connector
distributor for information about packaging collection and disposal policies in your
region.
1/34
English
10030119-12
Werbung
Inhaltsverzeichnis

Inhaltszusammenfassung für Siemens ACUSON AcuNav 8F
- Seite 1 Description 1. Inspect the connector for damage. Catheter ACUSON AcuNav 8F diagnostic ultrasound catheter 2. Inspect the sterile package and the catheter prior to use. ACUSON AcuNav 10F diagnostic ultrasound catheter 3. Using proper sterile technique, remove the catheter from the sterile package.
-
Seite 2: Указания За Употреба
За да подготвите катетъра и конектора за използване при Конвенция Описание ултразвуков преглед: Катетър ACUSON AcuNav 8F катетър за ултразвукова диагностика Проверете конектора за повреди. ACUSON AcuNav 10F катетър за ултразвукова диагностика Преди употреба проверете стерилната опаковка и катетъра. Конектор... - Seite 3 警告:如果包装已经打开或损坏,切勿使用该导管。使用保存在已打开或损 坏的包装中的导管,可能导致患者或用户受伤。请联系您的导管经销商。 警告:如果导管出现任何形式的损坏,切勿使用该导管。使用损坏的导管可 能导致患者或用户受伤。请联系您的导管经销商。 在您开始准备操作之前,请打开超声系统的电源。 要准备导管和连接器以便在超声检查中使用: 1. 检查连接器是否损坏。 约定 2. 在使用之前请检查无菌包装和导管。 约定 说明 3. 采用适当的无菌技法,从无菌包装中取出导管。将导管放在无菌工作区。 ACUSON AcuNav 8F 诊断用超声导管 导管 4. 检查整个导管是否损坏。 ACUSON AcuNav 10F 诊断用超声导管 5. 转动转向旋钮。转向功能应当平稳流畅。导管尖端应当在相应的方向上弯曲 达到 160°。 SwiftLink 导管连接器 连接器 注释:如果在您释放转向旋钮之后导管尖端没有返回中间位置,请确保张力 描述 控制旋钮被完全释放。逆时针方向彻底转动张力控制旋钮,以释放张力。 每只导管都是无菌的、一次性的,仅允许单次使用。ACUSON AcuNav 超声导 6. 将转向旋钮上的标记对准外壳上的标记,以使转向旋钮处于中间位置。...
-
Seite 4: Upute Za Rad
Priprema katetera i priključka za ultrazvučni pregled: Konvencija Opis 1. Provjerite je li priključak oštećen. Kateter Dijagnostički ultrazvučni kateter ACUSON AcuNav 8F 2. Prije uporabe provjerite sterilno pakiranje i kateter. Dijagnostički ultrazvučni kateter ACUSON AcuNav 10F 3. Primjenjujući odgovarajuću sterilnu tehniku, kateter izvadite iz sterilnog Priključak Priključak za kateter SwiftLink... -
Seite 5: Obecné Zásady
Obecné zásady Popis Příprava katétru a konektoru k použití pro ultrazvukové vyšetření: Katétr Diagnostický ultrazvukový katétr ACUSON AcuNav 8F 1. Zkontrolujte, zda není konektor poškozen. Diagnostický ultrazvukový katétr ACUSON AcuNav 10F 2. Před použitím zkontrolujte sterilní balení i katétr. Konektor Konektor katétru SwiftLink... - Seite 6 Sådan klargøres katetret og konnektoren til brug ved en Konvention Beskrivelse ultralydsundersøgelse: Kateter ACUSON AcuNav 8F diagnostisk ultralydskateter 1. Se konnektoren efter for tegn på skader. ACUSON AcuNav 10F diagnostisk ultralydskateter 2. Undersøg den sterile pakning og katetret før brug. Konnektor SwiftLink-kateterkonnektor 3.
-
Seite 7: Contra-Indicaties
De katheter en connector voorbereiden voor gebruik tijdens een Gebruik Beschrijving ultrasoon onderzoek: Katheter ACUSON AcuNav 8F diagnostische ultrasone katheter Inspecteer de connector op beschadigingen. ACUSON AcuNav 10F diagnostische ultrasone katheter Inspecteer de steriele verpakking en de katheter voorafgaand aan gebruik. Connector SwiftLink katheterconnector Gebruik de juiste steriele techniek om de katheter uit de steriele verpakking te nemen. - Seite 8 Enne ettevalmistusprotseduuride alustamist lülitage sisse ultrahelisüsteem. Lühend Kirjeldus Ultraheliuuringul kasutatava kateetri ja liitmiku ettevalmistamiseks toimige järgmiselt: Kateeter ACUSON AcuNav 8F’i diagnostiline ultrahelikateeter 1. Kontrollige liitmiku kahjustusnähtude suhtes. ACUSON AcuNav 10F’i diagnostiline ultrahelikateeter 2. Enne kasutamist kontrollige steriilset pakendit ja kateetrit. Liitmik SwiftLink kateetri liitmik 3.
- Seite 9 ESITYSTAVAT Valmistele katetri ja liitin ultraäänitutkimuksessa käyttöä varten Esitystapa Kuvaus seuraavasti: Katetri ACUSON AcuNav 8F diagnostinen ultraäänikatetri 1. Tarkista, ettei liitin ole vaurioitunut. ACUSON AcuNav 10F diagnostinen ultraäänikatetri 2. Tarkista steriili pakkaus ja katetri ennen käyttöä. Liitin SwiftLink-katetriliitin 3. Käytä asianmukaista steriiliä tekniikkaa ja poista katetri steriilistä...
-
Seite 10: Contre-Indications
Description Vérifiez que le connecteur ne soit pas endommagé. Cathéter Cathéter de diagnostic à ultrasons ACUSON AcuNav 8F Inspectez l’emballage stérile et le cathéter avant toute utilisation. Cathéter de diagnostic à ultrasons ACUSON AcuNav 10F Appliquez la technique stérile adéquate pour extraire le cathéter de l’emballage stérile. -
Seite 11: Anwendungsrichtlinien
Konvention Beschreibung Schalten Sie das Ultraschallsystem ein, bevor Sie mit den Vorbereitungen beginnen. Katheter Diagnostischer Ultraschallkatheter ACUSON AcuNav 8F So bereiten Sie den Katheter und den Anschluss für die Verwendung in Diagnostischer Ultraschallkatheter ACUSON AcuNav 10F einer Ultraschalluntersuchung vor: Anschluss SwiftLink-Katheteranschluss Überprüfen Sie den Anschluss auf Schäden. - Seite 12 Σύμβαση Περιγραφή Για να προετοιμάσετε τον καθετήρα και το σύνδεσμο για χρήση σε μια Καθετήρας Διαγνωστικός καθετήρας υπερήχων ACUSON AcuNav 8F εξέταση υπερήχων: Διαγνωστικός καθετήρας υπερήχων ACUSON AcuNav 10F Ελέγξτε το σύνδεσμο για τυχόν ζημιές. Πριν από τη χρήση, ελέγξτε τη στείρα συσκευασία και τον καθετήρα.
-
Seite 13: Használati Útmutató
Leírás Vizsgálja meg a csatlakozót sérülések szempontjából. Katéter ACUSON AcuNav 8F diagnosztikai ultrahangos katéter Használat előtt vizsgálja meg a steril csomagolást és a katétert. ACUSON AcuNav 10F diagnosztikai ultrahangos katéter Megfelelő steril technikát használva vegye ki a katétert a steril csomagolásból. - Seite 14 Konvensi Deskripsi Periksa apakah ada kerusakan pada konektor. Kateter Kateter ultrasonik diagnostik ACUSON AcuNav 8F Periksa kemasan steril dan kateter sebelum menggunakannya. Kateter ultrasonik diagnostik ACUSON AcuNav 10F Dengan menggunakan teknik steril yang benar, keluarkan kateter dari kemasan steril. Konektor Konektor kateter SwiftLink Tempatkan kateter di area kerja yang steril.
-
Seite 15: Istruzioni Per L'uso
Descrizione Ispezionare il connettore per verificare l’eventuale presenza di danni. Catetere Catetere a ultrasuoni per la diagnostica ACUSON AcuNav 8F Ispezionare la confezione sterile e il catetere prima dell’uso. Catetere a ultrasuoni per la diagnostica ACUSON AcuNav 10F Utilizzando una tecnica sterile appropriata, rimuovere il catetere dalla confezione sterile. - Seite 16 連絡してください。 警告: 包装が開封されていたり、破損しているカテーテルは使用しないでください。開 封されていたり、破損している包装に保管されていたカテーテルを使用すると、患者また はユーザーの負傷をまねくおそれがあります。カテーテルの販売代理店に連絡してくださ い。 警告: なんらかの損傷が認められるカテーテルは使用しないでください。破損しているカ テーテルを使用すると、患者またはユーザーの負傷をまねくおそれがあります。カテーテル の販売代理店に連絡してください。 凡例 準備手順を始める前に、超音波装置の電源を入れてください。 凡例 説明 カテーテルおよびコネクタを超音波検査に使用する準備をするには: ACUSON AcuNav 8F 診断用超音波カテーテル カテーテル 1. コネクタに損傷がないか点検します。 ACUSON AcuNav 10F 診断用超音波カテーテル 2. 使用前に滅菌包装およびカテーテルを点検します。 SwiftLink カテーテルコネクタ コネクタ 3. 適切な無菌操作を使用し、カテーテルを滅菌包装から取り出します。滅菌した作業区域に カテーテルを置きます。 説明 4. カテーテル全体に損傷がないか点検します。 各カテーテルは、滅菌済みのディスポーザブルで、単回使用についてのみライセンスされてい 5. ステアリングノブを回します。ステアリング機能は円滑でなければなりません。カテーテルチップ...
- Seite 17 검사 준비를 시작하기 전에 초음파 시스템의 전원을 켜십시오. 규칙 설명 초음파 검사에서 사용할 카테터 및 커넥터를 준비하려면 다음과 같이 하십시오: ACUSON AcuNav 8F 진단용 초음파 카테터 1. 커넥터에 손상이 있는지 검사합니다. 카테터 ACUSON AcuNav 10F 진단용 초음파 카테터 2. 사용하기 전에 카테터의 멸균 포장 상태를 점검합니다.
-
Seite 18: Lietošanas Norādījumi
Lai sagatavotu katetru un savienotāju lietošanai ultraskaņas Termini Apraksts izmeklēšanā: Katetrs ACUSON AcuNav 8F diagnostiskais ultraskaņas katetrs 1. Pārbaudiet, vai savienotājā nav bojājumu. ACUSON AcuNav 10F diagnostiskais ultraskaņas katetrs 2. Pirms lietošanas pārbaudiet sterilo iepakojumu un katetru. Savienotājs SwiftLink katetra savienotājs 3. -
Seite 19: Naudojimo Nurodymai
Lithuanian/Lietuviškai ACUSON „AcuNav“ ultragarsinis kateteris Paruošimo procedūra Etiketėje ant kateterio, jungties pateikiami simboliai ir naudojimo nurodymai ĮSPĖJIMAS: nenaudokite jungties, jei atrodo, kad ji bet kokiu būdu pažeista. aprašomi ACUSON „AcuNav“ ultragarsinio kateterio vartotojo vadove. Naudojant pažeistą jungtį galima sužeisti pacientą arba vartotoją. Kreipkitės į jungties platintoją. - Seite 20 Slå på ultralydsystemet før du starter forberedelsene. Konvensjon Beskrivelse For å klargjøre kateteret og kontakten til bruk i en ultralydundersøkelse: Kateter ACUSON AcuNav 8F diagnostisk ultralydkateter 1. Inspiser kontakten for skade. ACUSON AcuNav 10F diagnostisk ultralydkateter 2. Kontroller den sterile pakningen og kateteret før bruk. Kontakt SwiftLink kateterkontakt 3.
-
Seite 21: Wskazówki Dotyczące Użytkowania
Przygotowanie cewnika i łącznika do użycia podczas badania Przyjęta nazwa Opis ultradźwiękowego: Cewnik Diagnostyczny cewnik ultradźwiękowy ACUSON AcuNav 8F Sprawdź, czy łącznik nie jest uszkodzony. Diagnostyczny cewnik ultradźwiękowy ACUSON AcuNav 10F Przed użyciem wzrokowo sprawdź sterylne opakowanie i cewnik. Łącznik Łącznik cewników SwiftLink... -
Seite 22: Instruções De Utilização
Para preparar o cateter e conector para utilização num exame por ultra- Convenção Descrição sons: Cateter Cateter ultrassónico de diagnóstico ACUSON AcuNav 8F 1. Inspeccione o conector para detectar danos. Cateter ultrassónico de diagnóstico ACUSON AcuNav 10F 2. Inspeccione a embalagem estéril e o cateter antes da sua utilização. Conector Conector de cateter SwiftLink 3. - Seite 23 Para preparar o cateter e o conector para uso em um exame de ultra- sonografia: Cateter Cateter ultrassônico de diagnóstico ACUSON AcuNav 8F 1. Inspecione o conector à procura de danos. Cateter ultrassônico de diagnóstico ACUSON AcuNav 10F 2. Inspecione a embalagem estéril e o cateter antes do uso.
-
Seite 24: Recomandări De Utilizare
Pentru a pregăti cateterul şi conectorul pentru utilizarea în cadrul unei Convenţia Descriere investigaţii cu ultrasunete: Cateter Cateter de diagnosticare prin ultrasonografie ACUSON AcuNav 8F Inspectaţi conectorul pentru semne de deteriorare. Cateter de diagnosticare prin ultrasonografie ACUSON AcuNav 10F Inspectaţi ambalajul steril şi cateterul înainte de utilizare. Conector Conectorul pentru cateter SwiftLink Utilizând o tehnică... -
Seite 25: Условные Обозначения
Проверьте, не поврежден ли соединитель. обозначение Описание Перед использованием осмотрите стерильную упаковку и катетер. Катетер Диагностический ультразвуковой катетер ACUSON AcuNav 8F Подходящим асептическим методом извлеките катетер из стерильной упаковки. Диагностический ультразвуковой катетер ACUSON AcuNav 10F Положите катетер на стерильную рабочую поверхность. Соединитель... -
Seite 26: Uputstvo Za Upotrebu
Konvencija Opis Da biste pripremili kateter i priključak za upotrebu za ultrazvučni pregled: Kateter ACUSON AcuNav 8F dijagnostički ultrazvučni kateter 1. Proverite da priključak nije oštećen. ACUSON AcuNav 10F dijagnostički ultrazvučni kateter 2. Pre upotrebe proverite sterilno pakovanje i kateter. - Seite 27 Postup pri príprave ultrazvukového katétra a konektora na použitie pri ultrazvukovom vyšetrení: Katéter Diagnostický ultrazvukový katéter ACUSON AcuNav 8F 1. Skontrolujte, či konektor nie je poškodený. Diagnostický ultrazvukový katéter ACUSON AcuNav 10F 2. Pred samotným použitím skontrolujte sterilný obal a katéter.
- Seite 28 Kateter in spojnik pripravite za uporabo pri ultrazvočni preiskavi, in Izraz Opis sicer: Kateter Diagnostični ultrazvočni kateter ACUSON AcuNav 8F 1. Preverite, ali je kateter poškodovan. Diagnostični ultrazvočni kateter ACUSON AcuNav 10F 2. Pred uporabo preverite sterilni zavoj in kateter. Priključek Katetrski spojnik SwiftLink 3.
-
Seite 29: Instrucciones De Uso
Descripción 1. Revise el conector para determinar si está dañado. Catéter Catéter ecográfico de diagnóstico ACUSON AcuNav 8F 2. Revise el empaque estéril y el catéter antes del uso. Catéter ecográfico de diagnóstico ACUSON AcuNav 10F 3. Mediante una técnica estéril apropiada, retire el catéter del empaque estéril. - Seite 30 Användning av en skadad kateter kan leda till skador på patienten eller användaren. Kontakta istället kateterdistributören. Benämning Beskrivning Slå på ultraljudssystemet innan du sätter igång med förberedelserna. Kateter ACUSON AcuNav 8F diagnostisk ultraljudskateter Förberedelse av kateter och kateteranslutning för användning vid ACUSON AcuNav 10F diagnostisk ultraljudskateter ultraljudsundersökning: Kateteranslutning SwiftLink kateteranslutning 1.
-
Seite 31: Kullanim Tali̇matlari
Bir ultrason muayenesinde kullanılmak üzere kateterin ve konektörün Kural Açıklama hazırlanması için: Kateter ACUSON AcuNav 8F diagnostik ultrason kateteri 1. Konektörde herhangi bir hasar olup olmadığını kontrol edin. ACUSON AcuNav 10F diagnostik ultrason kateteri 2. Kullanmadan önce steril ambalajı ve kateteri inceleyin. Konektör SwiftLink kateter konektörü... - Seite 32 Trước khi quý vị bắt đầu các quy trình chuẩn bị, hãy bật máy siêu âm lên. Ống thông Ống thông dùng trong siêu âm chẩn đoán ACUSON AcuNav 8F Chuẩn bị ống thông và bộ dây nối để sử dụng trong khi kiểm tra trên Ống thông dùng trong siêu âm chẩn đoán ACUSON AcuNav 10F...
- Seite 33 10030119-ABS-001-12-12 33/34 10030119-12...
- Seite 34 91052 Erlangen documentation. Siemens does not assume responsibility for the Germany performance of third-party products. Siemens reserves the right to change its products and services at any time. In addition, this manual is subject to change without notice. Legal Manufacturer Siemens Healthcare Headquarters Siemens Medical Solutions USA, Inc.











